Alessandra Magistrato
Computational Chemistry & Biology


Recognition of DNA Damages by Inorganic Probes
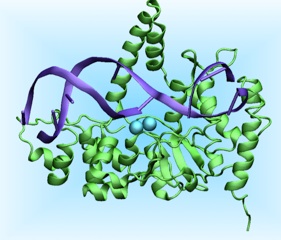
Genomic integrity is fundamental for cell life and survival. DNA damages and mutations occur frequently in the cells caused by several agents (ranging from genotoxic chemicals to error-prone cellular process). If left unrepaired these damages can lead to cancerous transformations. Metalloinsertors such as Δ-Rh[(bpy)2(chrysi)]3+ can selectively recognize a mismatch in the DNA with selectivity higher than 80%, irrespective of the DNA sequences around the mismatch. These molecules bind non covalently to DNA duplexes from the minor groove side with separation and displacement of the mismatched base pair and one ligand of the molecule acts as a π-stacking replacement in the DNA duplex (Pierre et al. Proc. Natl. Aacad. Sci. USA 2007). This newly discovered DNA binding mode is called insertion, hence the name metalloinsertors. These molecules have been demonstrated to selectively recognize mismatches with the following decreasing affinity CC>AC>AA. However, they can also selectively target single base bulges and abasic sites, although with lower affinity. Most importantly, Rh metalloinsertors have been recently demonstrated to act as chemotherapeutic agents in vivo in mismatch repair (MMR) deficient cell lines. (Ernst et al. J. Am. Chem. Soc. 2009)
Currently we are investigating via classical molecular dynamics (MD) simulations the structural features of the insertion binding mode trying to understand the reason of the different binding affinity towards different mismatches. In addition, we are studying the dissociation mechanism of metalloinsertors via path collective variable and metadynamics simulations. Preliminary studies suggest a multistep dissociation pathway with a barrier of 14 kcal/mol, in good agreement with experiments. Understanding the factors that tune the different affinity of metalloinsertors towards DNA bases as well as the principles of their recognition mechanism may be of crucial importance in the design of novel chemotherapeutics agents.
Research Activity
Dr. A. Magistrato is currently involved in studying biological processes that lead to the onset of human diseases (i.e. cancer, neurodegeneration, diabete). Many efforts are currently devoted to understand the molecular recognition principles of DNA damages. This important biological issue is addressed from different point of views:
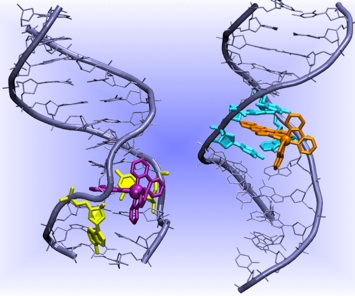
As stated above metalloinsertors bind to the minor groove, intercalating one aromatic ligand and expelling the mismatched bases. In contrast, Ru containing metallointercalators bind to the DNA major grove and posses at least a ligand that can intercalate and π-stack to DNA duplexes. However, they are unable to detect DNA mismatches. These Ru molecules possess solvatochromic properties that prove their interactions with specific DNA sequences. The factors tuning the different binding behavior between metallointercalators and insertors are unclear. Steric bulk of the intercalating/inserting ligand has been pointed out as the most likely candidate, but experiments have shown that it is not the only important factor (Zeglis et al. Inorg. Chem. 2008). Therefore, we are focusing on archetypal examples of metallointercalators and metalloinsertors to understand the structural, electronic properties dictating their different binding mode to DNA. In particular, we have considered Δ-Ru(bpy)2(ddpz)2+ and Δ-Rh(bpy)2(chrysi)3+ as a prototypes of metallointercalators and metalloinsertors, respectively. These studies are currently performed via classical and mixed quantum-classical (QM/MM) MD simulations.
Recognition of DNA Damages by Repair Enzymes.
DNA damages can be provoked by endogenous and exogenous sources such as replication errors or alkylating agents, oxygen radicals, UV light and X-rays. The recognition and the repair of these damages is a key-process for preventing the development of several diseases, including cancer. Multiple and diverse pathways exist to repair DNA-lesions. In particular, in the base excision repair pathway (BER), DNA damages resulting from deamination, oxidation and alkylation are detected and repaired by the coordinate effort of a set of specialized enzymes. One of the most conserved enzyme taking part to this process is Uracil DNA Glycosylase (UDG), which removes the uracil from DNA. This prevents C to T transition mutations, which arise from cytosine deamination. One intriguing aspect of UNG is an extra helical recognition mechanism whereby the uracil is expelled from the DNA base stack and engulfed into the active site pocket of the enzyme, where, subsequently, the cleavage of the uracil base occurs. In analogy with other BER enzymes, crystallographic as well as spectroscopic measurements have elucidated that the base flipping mechanism of UDG is a multistep process (Parker et al. Nature 2007).
However, a detailed understanding of the flipping mechanism is still lacking as only some of the intermediates of this pathway have been identified. Starting from the two crystallographic structures of a mutant of the DNA-UNG complex with a completely and a partially flipped uracil, we will are currently investigating the flipping pathway of the DNA-UDG adduct. We are using metadynamics, in a classical MD framework, to elucidate the complex free energy landscape of the uracil flip. This will provide also a picture at atomistic level of the reaction intermediates that have been detected only by spectroscopic and kinetic measurements (Stivers J. Chem. Eur. J. 2008). In addition, a comparison with the other BER enzymes, which recognize other DNA damages via a flipping mechanism, will allow to generalize the principles at the basis of this crucial biological process.
Enzymatic Catalysis
Hormones Biosynthesis in Human Aromatase
Aromatase cytocrome P450 is an iron containing enzyme of crucial biological and pharmacological relevance as is the only enzyme in vertebrates to catalyze the biosynthesis of all oestrogens from androgens. Aromatase inhibitors are, therefore, of paramount importance in the therapy of estrogen dependent breast cancer. Despite the importance of this enzyme, its membrane bound nature has prevented its crystallization for many years. This problem has been addressed only recently and the new crystal structure has provided the structural information concerning the location of key residues of the active site and of an inactive substrate (Ghosh et al. Nature 2009). This information is fundamental to shed light on the catalytic mechanism of this enzyme. The enzymatic process proceeds in three steps, each step requiring 1 mol of O2 and 1 mol of NADPH and, coupling with its redox partner, aromatase converts androstenedione, testosterone, and 16α-hydroxytestosterone to oestrone, 17β-oestradiolo and 17β,17α-oestriol, respectively.
The first two steps are C19 methyl hydroxylation steps, while the third involves the aromatization of the A ring of the substrate, which is unique to aromatase. The way in which aromatase catalyzes this latter step is still highly debated. In addition, the enzyme presents a high androgenic specificity at variance with other P450 cytochromes, whose reasons are not fully clear. We are currently investigating the mechanism of aromatase via classical and mixed QM/MM MD simulations to understand its unique catalytic mechanism and the molecular basis for its high androgenic specificity. This information may be of help for developing next generation aromatase inhibitors to be employed in anticancer therapy.
The transport mechanism of sodium-coupled galactose symporter
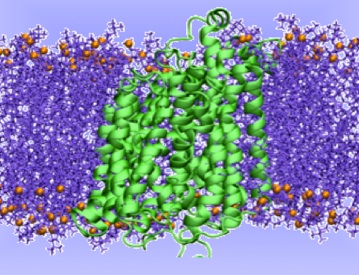
This has provided the basis for a rational understanding of the mechanism of the transport cycle, hypothesized to occur via an alternating-access model, but a detailed atomistic picture of the structural changes necessary to accomplish the transport of the substrate are still unclear, as well as a detailed thermodynamic and kinetic characterization of each step of the transport cycle.In this project we aim at investigating the structural, thermodynamic and kinetic properties of the transport mechanism of a prokaryotic member of the SSS family, the Vibrio parahaemolyticus Na+/galactose transporter, vSGLT. We intend to address this issue by performing all-atom explicit-solvent molecular dynamics simulation in combination with bias-exchange metadynamics, an efficient computational approach to simulate rare events.
Neurodegenerative Diseases: The role of metals in Alzheimer’s Disease



Mechanism of Flap Endonucleases (FEN)
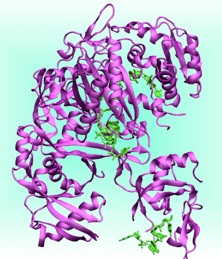
Flap Endonucleases (FEN) are essential for DNA replication and repair as they remove RNA and DNA 5’-flaps using a divalent metal ion (Mg2+) dependent activity. FEN play an essential role for life as DNA replication generates 50 million Okazaki’s primers at each cell cycle. Failure to remove precisely these primers create gaps, or overlaps that interfere with cell division and with the maintenance of genome fidelity. We are currently focusing on the human Flap endonuclease 1 (hFEN), an enzyme over expressed in all proliferative tissues, which has been recently proposed as a potential target for chemotherapy.
Recently, the X-ray structures of hFEN/substrate and product complexes were solved. Notably, the structure of hFEN/substrate shows the scissile phosphate, undergoing the cleavage reaction, far from the catalytic site. Therefore, essential information to study the reactivity of this enzyme are lacking. These information may be of crucial importance for drug design. In contrast, the structure of hFEN/product provides structural details on the position of the scissile phosphate at the end of the catalytic reaction. We are currently attempting at providing these structural information by computational modeling with the aim of characterizing in detail the reaction mechanism of hFEN.
Gene Silencing by Argonaute Proteins
Argonautes (AGO) form an evolutionarily conserved family of proteins whose members silence gene expression in pathways such as RNA interference (RNAi). In eukariotes these proteins bind small RNAs (guide strand) and use their sequence to target mRNAs via sequence complementarity. Argonaute–small-RNA complexes can repress the transcription of genes, target mRNAs for site-specific cleavage or general degradation, or block mRNA translation into protein. AGO proteins bind ∼21 nt small interfering RNAs (siRNAs) and 21–23 nt microRNAs (miRNAs). Some AGO proteins posses slicer activity mediated by Mg2+ ions and promote silencing by endonucleolytic cleavage of its RNA target at a single phosphodiester bond. Terefore Ago is the catalytic component of the RNA silencing. Others do not posses this slicer activity however all of them mediate silencing activity by translational repression or adenylation. This process interference with crucial biological processes such as development. cancer, genome maintenance. The recent crystallization of human Ago-2 leads to exciting advances into the understanding of eukariotic RNAi. Thank to molecular simulation tools ranging from bioinformatic to classical MD and hybrid QM/MM MD we are identifying the Mg2+ binding sites in Ago-2 and determinimg the structure of the complex between Ago-2 and dsRNS with the aim of elucidating the mechanism of human Ago proteins.
Sodium-coupled transporters use the energy stored in Na+ gradients for an active accumulation of nutrients into the cells. Among these, solute-sodium symporters (SSS) are a large family of proteins that cotransport Na+ together with sugars, aminoacids, inorganic ions or vitamins. In particular, the Na+-glucose (galactose) symporters drive the cellular uptake of sugar in many organs of the human body. These proteins are involved in the onset of different diseases. For example, the human SGLT (hSGLT) symporters are the target for oral rehydration therapy and for type II diabetes. Furthermore, glucose demand in cancer cells is higher than in normal cells and, therefore, these types of symporters play an important role also in cancer. Several crystal structures have been recently solved for different members of the SSS family, corresponding to different stages of the substrate transport cycle.
Alzheimer's Disease (AD) is the most common form of dementia in the elderly population. It impairs memory and thinking, ultimately leading to death. More than 30 million people worldwide suffer from this disease for which a cure does not yet exists. AD is characterized by the presence of intracellular neurofibrillary tangles and extracellular amyloid plaques composed by Aß aggregated forms (e.g., oligomers, protofibrils, fibrils). Aß is a peptide of 39-43 amino acids. The mechanism leading to the formation of Aß aggregates is not clear yet. Extensive laboratory studies indicated that the morphology and kinetics of formation of Aß aggregates is affected by many factors, including the concentration of metals.
In particular, metal ions (Cu(II), Zn(II) and Fe(II)) were found at particularly high concentrations in senile plaques, suggesting that an altered metal metabolism or a particularly high concentration of these metals in selected brain regions may be associated to the neurodegenerative process. Many experimental evidences highlighted the association of metal ions with AD, leading to the postulate that compounds capable of inhibiting metal/Aß interactions and redistribute the metal ions may offer a therapeutic potential against AD. These molecules are classified as bifunctional compounds (BCs), since they can directly interact with Aß (even in absence of metals) and chelate the metals.
By means of an integrated spectroscopic and modeling approach we intend to elucidate the mode of action of these compounds. In doing so, we will have also to address the structure of the Cu(II)-Aß adduct itself, still controversial in the literature. Attainment of structural models of the Cu(II)-Aß adduct will allow to further advance the understanding of the factors driving Aß associations with Cu(II).
